An Aging Reset Button: Rejuvenation Through Apheresis
Over the last century, the human lifespan has increased dramatically due to the rise of modern medicine. Antibiotics, vaccines, and countless other technologies have added years onto our pre-scientific revolution lives. Unfortunately, we may have reached a tipping point.
While healthcare has advanced far enough that we could theoretically live well into our 100s, the lack of tangible breakthroughs concerning age-related diseases means that we are not able to reach our maximum living potential.
Aging is an highly complex biological process; thousands of different physical, environmental, and external factors drive it forward or slow it down. Since aging is such a multi-dimensional issue, there is likely no single silver bullet solution to the equation.
To solve the aging problem, we must stop the process at the source. If we can nip the issue in the bud, the human lifespan could push well past the point of meeting your great-great-great-grandchildren.
Three significant processes cause the aging pistons to fire: chronic inflammation, immunosenescence (i.e., aged immune cells), and cellular senescence (i.e., zombie cells, which accelerate aging). These three pillars are likely some of the leading underlying causes driving the age-related incidence of diseases such as cancer, arthritis, Alzheimer’s, and many other conditions.
In essence, these three processes shift the body away from operating in a typical regulated state (homeostasis) and push it towards an always-on alert mode where every defensive asset is in overdrive. In turn, this leads to a hyper pro-inflammatory immune system which wreaks havoc and malfunctions over time, making the body more prone to develop conditions such as strokes or heart attacks.
So now that we know that these aging drivers cause overexpression of pro-inflammatory molecules in the body, how do we fix this? How do we remove these molecules?
Interestingly, a striking and relatively old concept known as parabiosis has found its way back into the limelight. The Greek word derives meaning from the prefix para, ‘alongside,’ and the suffix bios, ‘life,’ and has shown potential as a reversal or delaying method for the trio of quintessential aging hallmarks.
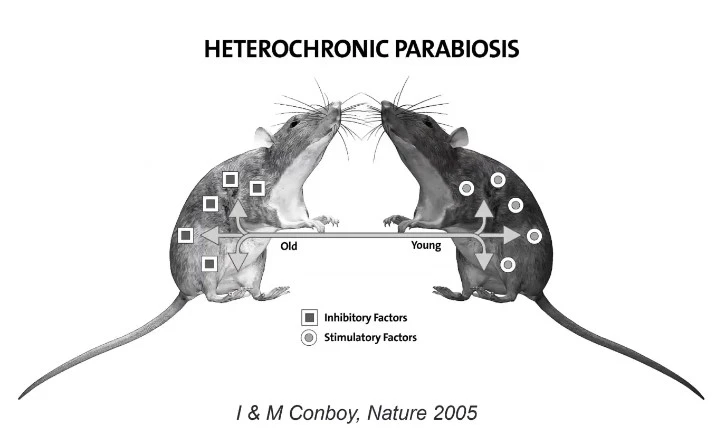 In a seminal paper published by Irina Conboy and Michael Conboy in 20051, two genetically identical mice were used to demonstrate this concept. In their research, the two mice (one old and one young mouse) had their circulatory systems connected in a fashion that both lived off of the same general blood pool, a combination of both young and old blood.
In a seminal paper published by Irina Conboy and Michael Conboy in 20051, two genetically identical mice were used to demonstrate this concept. In their research, the two mice (one old and one young mouse) had their circulatory systems connected in a fashion that both lived off of the same general blood pool, a combination of both young and old blood.
This experiment suggested that the exposure of the older progenitor to the blood of the younger offspring restored portions of the elder’s body function. Within five weeks, the young blood caused aged stem cells to start dividing again, repairing muscle and liver cells in the older mouse. Essentially, the older mouse became younger from a cellular point of view.
Shortly after Conboy squared’s work, additional research showed that young plasma (the liquid part of the blood fluid that contains proteins) alone was sufficient to provide rejuvenating effects.
Fortunately for us, we can cut the vampiric blood swap from the equation.
Since the advent of this work, researchers have been exploring the use of Therapeutic Plasma Exchange (TPE), or plasmapheresis, to replicate the results shown by the Conboys and mitigate the effects of aging in humans.
TPE is not a new therapy; this procedure has been around since the 1980s and is validated and approved to treat more than 100 different conditions (although typically reserved to treat relatively uncommon life-threatening illnesses).
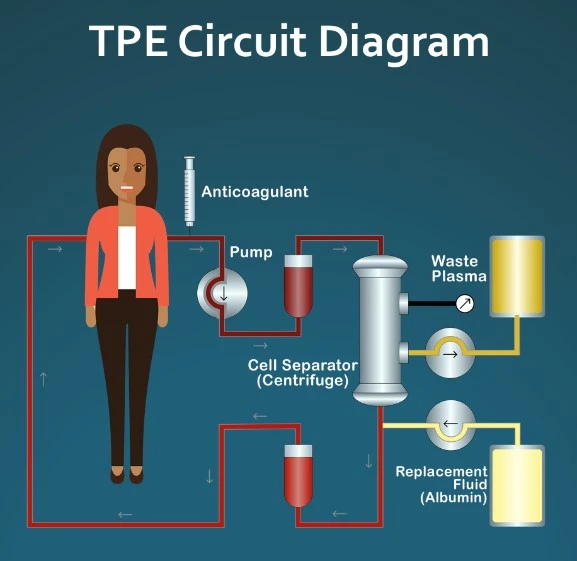 The TPE procedure involves passing a person’s blood through an apheresis machine that separates their plasma, discards it, and reinfuses them with a combo of their old blood cells along with a replacement solution (typically plasma, but other solutions can be used).
The TPE procedure involves passing a person’s blood through an apheresis machine that separates their plasma, discards it, and reinfuses them with a combo of their old blood cells along with a replacement solution (typically plasma, but other solutions can be used).
Even though using fresh plasma from a young donor may sound like the ideal choice, there are several logistical and medical issues involved. Plasma infusions are occasionally associated with dangerous adverse effects, such as anaphylactic reactions, and the process of storing/transporting frozen plasma is cumbersome and costly. With these in mind, using fresh frozen plasma would essentially limit this procedure to hospital settings only.
Luckily, there may be another option.
Recently, there have been encouraging regenerative results for people undergoing TPE replacements with a different plasma exchange cocktail.
In particular, replacing old plasma with saline (salt and water) containing albumin, a blood serum protein, has been shown to slow the aging process while mitigating the issues with donor plasma. It is uncertain if the key for regeneration lies in albumin administration or just the dilution of “old” pro-inflammatory factors contained in the person´s blood.
While the jury is still out on that, one prospect is clear: if saline-albumin transfers can sufficiently change the body’s systemic milieu, TPE could serve as a molecular reset button to treat some of the hallmarks of aging.
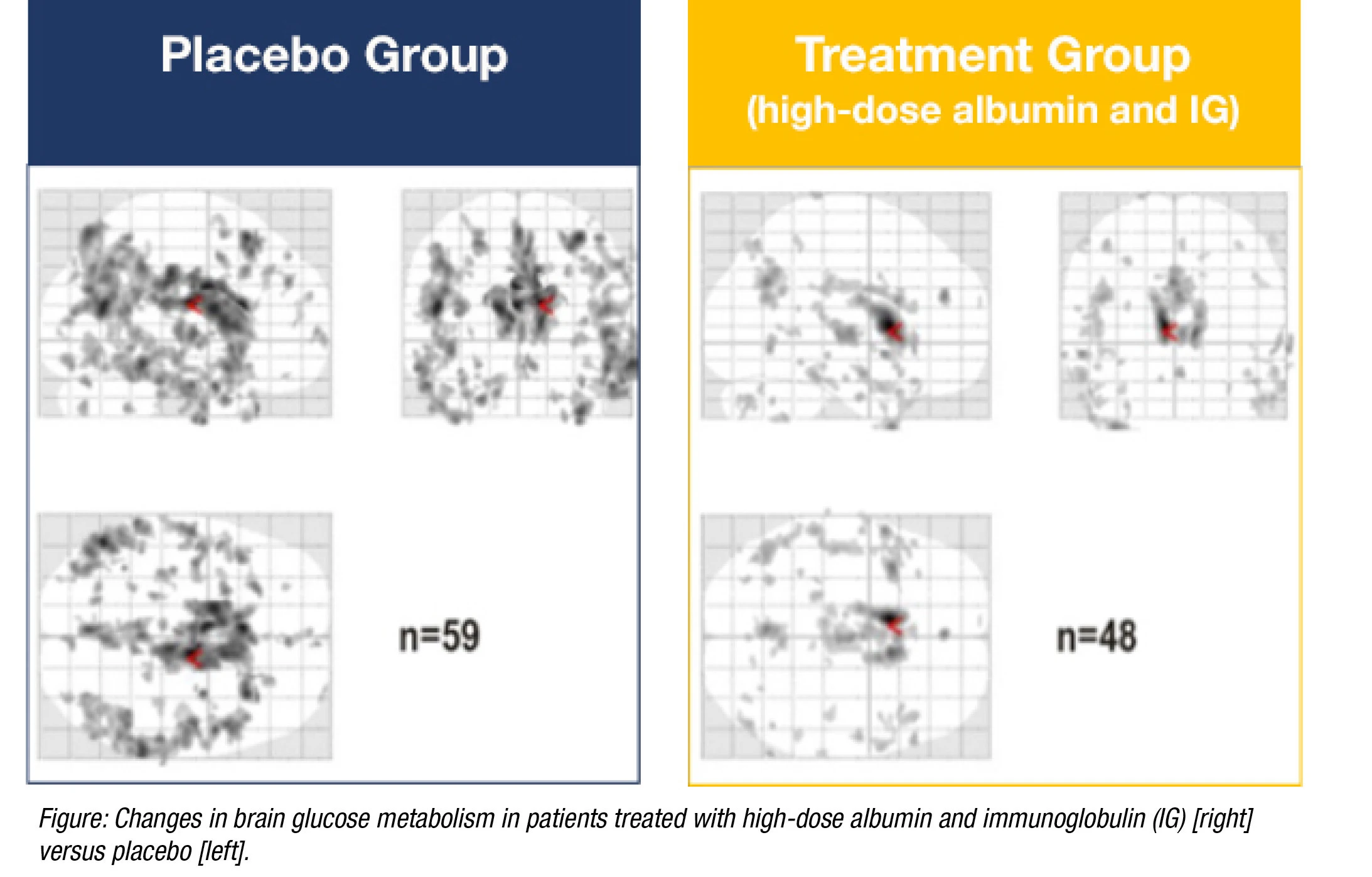
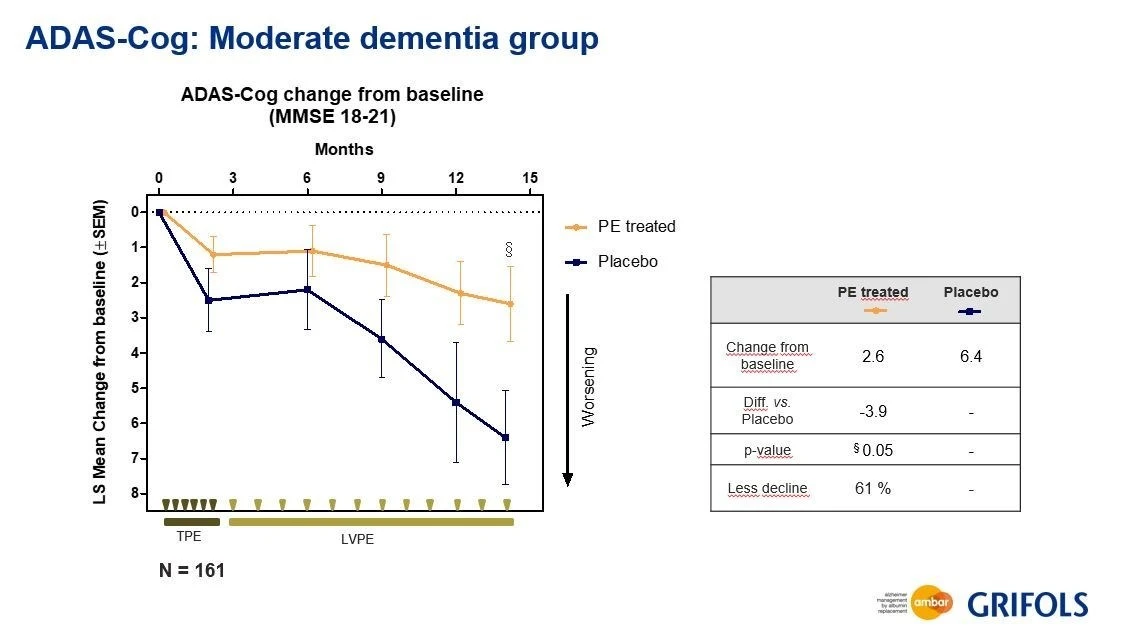
Several clinical trials are working to demonstrate the positive effects of saline-albumin transfers. One remarkable study, AMBAR (Alzheimer Management by Albumin Replacement), is a randomized clinical trial that has recruited more than 400 Alzheimer’s patients with the intent to delay their cognitive decline through the use of TPE.
This trial has shown potential preventive effects by improving cognitive function and increasing mental fortitude. Even though Alzheimer’s is a complex disorder with an unknown ultimate cause, high safety last-shot therapies such as TPE could have unlimited upside and minimal downside for patients in this demographic.
Working to pin down precise repair mechanisms could be one key to preventing age-related decline and increasing human lifespan. As we look forward into the twenty-first century’s third decade, TPE’s combination of a quick offset, efficacious safety profile, and profound regenerative effects could position it to be one of the major rejuvenating modalities of our future.